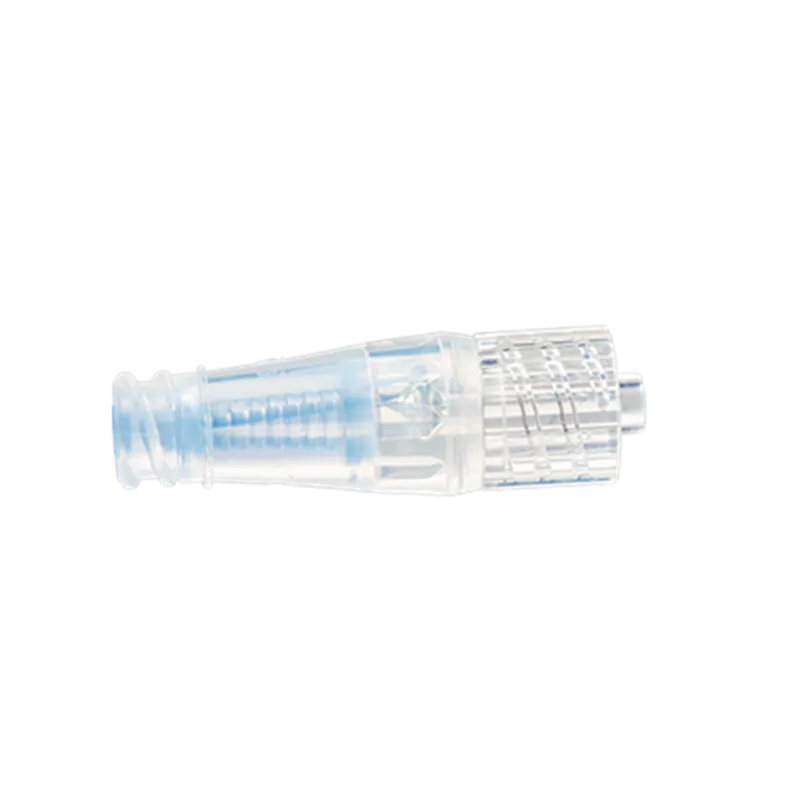
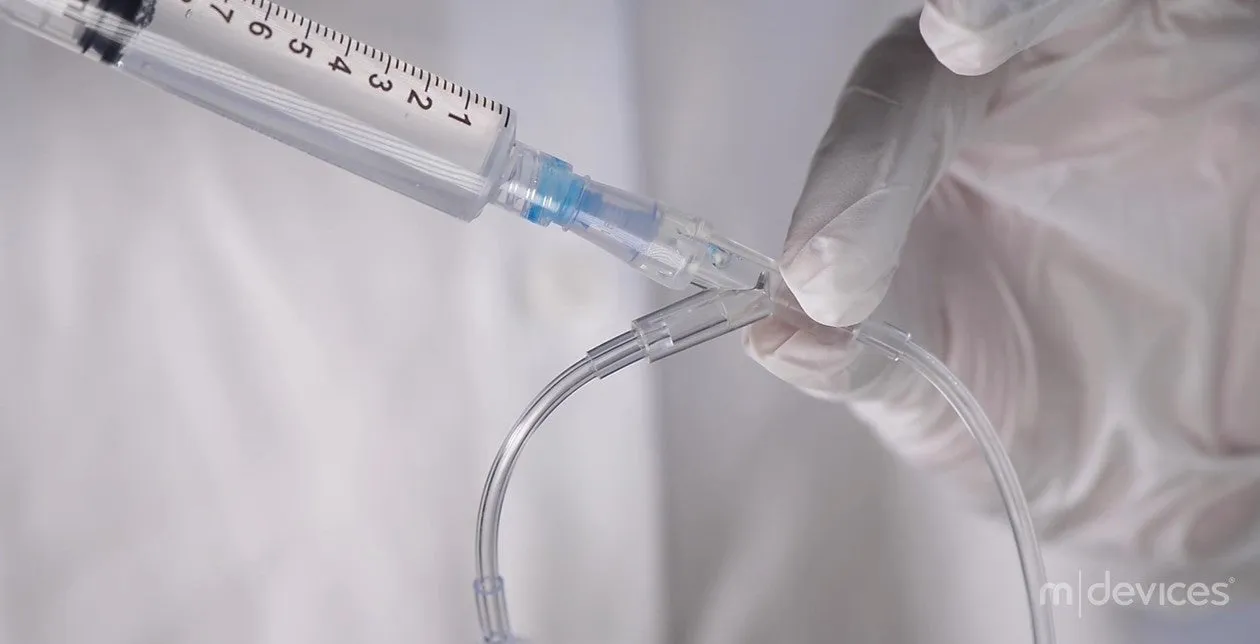
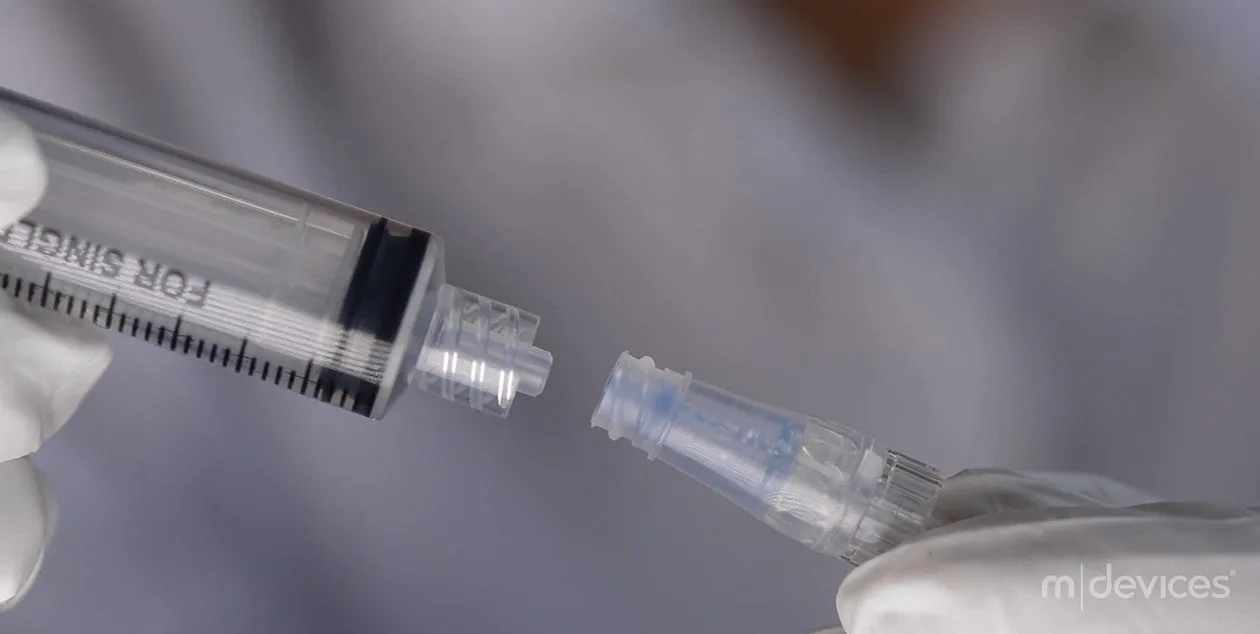
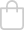
Introducing the m|devices NeutralSite™ Valve, a cutting-edge solution designed to enhance catheter care and reduce the risk of complications. This innovative medical device is engineered with precision to provide optimal performance and safety. The NeutralSite™ Valve is a crucial component in catheter management, addressing key concerns such as catheter occlusions and bloodstream infections. Its advanced features and thoughtful design make it a reliable choice for healthcare professionals seeking excellence in patient care.
Features:
- Flat Swabable Pre-Slit Septum: Easy and efficient disinfection with a flat surface and pre-slit septum.
- Straight Internal Fluid Pathway: Minimises priming volume and addresses dead space concerns for enhanced fluid management.
- Low Priming Volume: Efficiently reduces the amount of fluid needed for priming.
- Microbial Barrier: Gapless space between septum and casing acts as a microbial barrier, preventing contamination.
- Lipid Resistant: Resistant to lipids, ensuring durability and longevity.
- Transparent Housing: Clear visibility during flushing for added convenience.
- Compatibility: Works seamlessly with both luer lock and luer slip connectors.
- Sterile: Ensures a sterile environment for medical procedures.
Specifications:
- Neutral Pressure Displacement: Provides neutral pressure displacement with minimal reflux volume.
- Maximum Flow Rate at 350psi: 10mL/second for efficient fluid management.
- Number of Accesses: Designed for over 200 accesses, ensuring durability.
- Priming Volume: Only 0.08mL required for priming.
- Reflux Volume: Minimal reflux volume of 0.0059mL.
- PSI Rating: Sturdy construction with a PSI rating of 350psi.
- Biological Evaluation: Compliant with EN ISO 10993 standards for biological evaluation.
- Lipid Resistance: Successfully tested as lipid-resistant.
- ARTG Number: Registered with ARTG as number 355439.
- Material: Constructed with PP, ABS, and silicone for reliability.
- MRI Compatible: Safe for use in MRI environments.
Clinical Validation:
The NeutralSite™ Valve underwent rigorous testing, demonstrating its effectiveness in preventing bacterial contamination. In a 7-day study with 140 activations, the valve achieved a bacterial log reduction of 7.
Usage Guidelines:
- For optimal results, the NeutralSite™ Valve is recommended for use by qualified clinicians, following facility protocols for all clinical procedures.
- Enhance catheter care with the m|devices NeutralSite™ Valve – a testament to innovation, reliability, and patient safety.
Note: Recommended use of up to 7 days as per independent laboratory test study.
Resources: